 研发追踪
研发追踪
 复宏汉霖
复宏汉霖  2026-01-13
2026-01-13
 65
65
2026年美国临床肿瘤学会胃肠道肿瘤研讨会(ASCO GI)于1月8日至10日在美国旧金山举行。在本次大会上,复宏汉霖创新型抗PD-1单抗H药 汉斯状®在MSS/pMMR局部晚期结/直肠癌领域的两项新辅助研究结果以壁报形式正式发布,进一步彰显了H药在消化道肿瘤领域的治疗潜力。H药是全球首个且唯一胃癌围手术期III期注册研究成功的抗PD-1单抗,也是全球首个一线治疗小细胞肺癌的抗PD-1单抗,在肺癌和胃癌领域皆斩获全球突破性进展。
在消化道肿瘤治疗领域,H药除已获批食管鳞状细胞癌(ESCC)外,更是首个获得国家药品监督管理局(NMPA)药品审评中心(CDE)突破性疗法认定的胃癌围手术期治疗药物。作为全球首个胃癌围手术期以免疫单药取代术后辅助化疗的治疗方案,该适应症上市许可申请已获CDE受理并被纳入优先审评。此外,斯鲁利单抗联合贝伐珠单抗联合化疗一线治疗转移性结直肠癌(mCRC)的国际多中心临床研究(ASTRUM-015)III期研究已完成患者入组,有望填补一线免疫治疗non-MSI-H mCRC的临床空白。在本次ASCO GI大会上,H药公布了两项针对局晚期结/直肠癌的新辅助治疗IIT研究积极结果。数据显示,H药用于新辅助治疗有望提升局晚期高危结肠癌患者的根治性手术机会与长期生存率,同时也展现出为局晚期直肠癌患者提供免放疗治疗方案的潜力。
凭借其差异化的机制,H药在多种实体瘤的治疗中展现出独特优势,该药物不仅具备更强的PD-1内吞作用,可减少T细胞表面PD-1受体[1],实现快速、强效的免疫激活;还能减少PD-1对共刺激分子CD28的募集,从而更大程度保留CD28信号传导[2-4],增强下游AKT蛋白活性[5],促进T细胞持续活化。聚焦消化道肿瘤与肺癌,H药已获批用于治疗鳞状非小细胞肺癌(sqNSCLC)、广泛期小细胞肺癌(ES-SCLC)、ESCC和非鳞状非小细胞肺癌(nsNSCLC) 适应症,在中国、英国、德国、新加坡、印度等40多个国家获批上市,覆盖全球近半数人口。其关键性临床研究结果发表于JAMA、Nature Medicine、Cancer Cell和British Journal of Cancer等顶级学术期刊,并获得美国、欧盟、瑞士、韩国、墨西哥孤儿药资格认定。
此次ASCO GI大会上发布的两项研究数据结果如下:
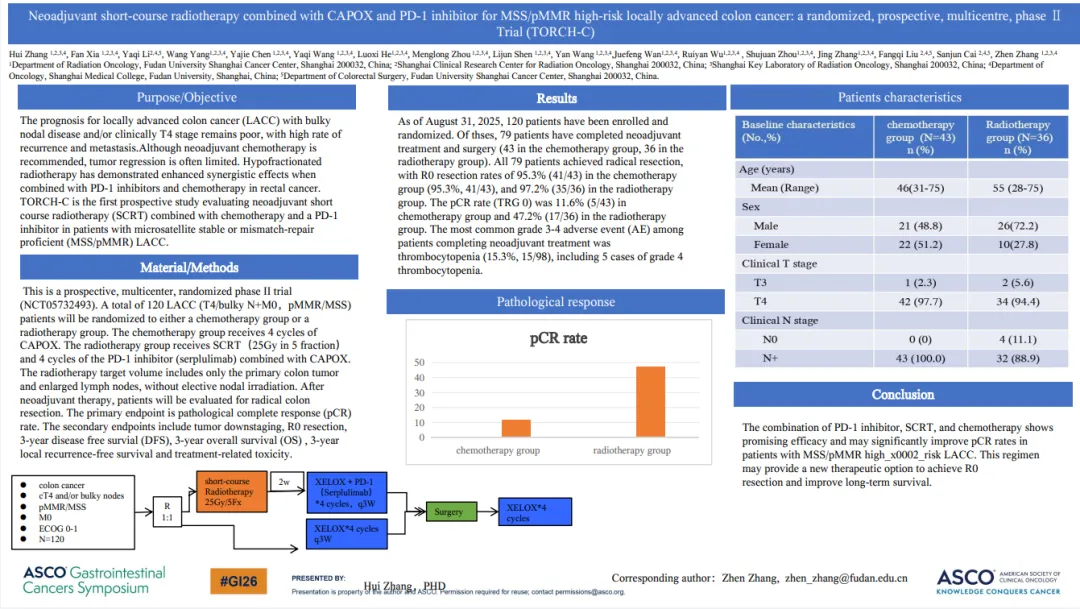
新辅助短程放疗联合 CAPOX 方案与 PD-1 抑制剂用于 MSS/pMMR 高危局部晚期结肠癌:一项随机、前瞻性、多中心 Ⅱ 期试验(TORCH-C)
试验设计:
这是一项前瞻性、多中心、随机II期试验(NCT05732493)。总共120例LACC (T4/大体积N+M0,pMMR/MSS)患者将随机分为化疗组或放疗组。化疗组给予4个周期的CAPOX。放疗组接受5次25Gy的SCRT治疗,PD-1抑制剂(斯鲁利单抗)联合CAPOX治疗4个周期。放疗靶体积仅包括原发结肠肿瘤和肿大的淋巴结,没有选择性淋巴结照射。在新辅助治疗后,患者将评估根治性结肠切除术。主要终点是病理完全缓解(pCR)率。次要终点包括肿瘤降期,R0切除,3年无病生存期(DFS),3年总生存期(OS),3年局部无复发生存期和治疗相关毒性
结果:
截至2025年8月31日,已有120名患者入组并随机分组。其中79例患者完成了新辅助治疗和手术(化疗组43例,放疗组36例)。79例患者均获得根治性切除,化疗组R0切除率为95.3%(41/43),放疗组R0切除率为97.2% (35/36)。化疗组pCR率(TRG 0)为11.6%(5/43),放疗组为47.2%(17/36)。完成新辅助治疗的患者中最常见的3-4级不良事件(AE)是血小板减少(15.3%,15/98),其中4级血小板减少5例。
结论:
PD-1抑制剂、SCRT和化疗联合应用效果良好,可显著提高MSS/pMMR高危LACC患者的pCR率。该方案可能为实现R0切除和提高长期生存率提供新的治疗选择。
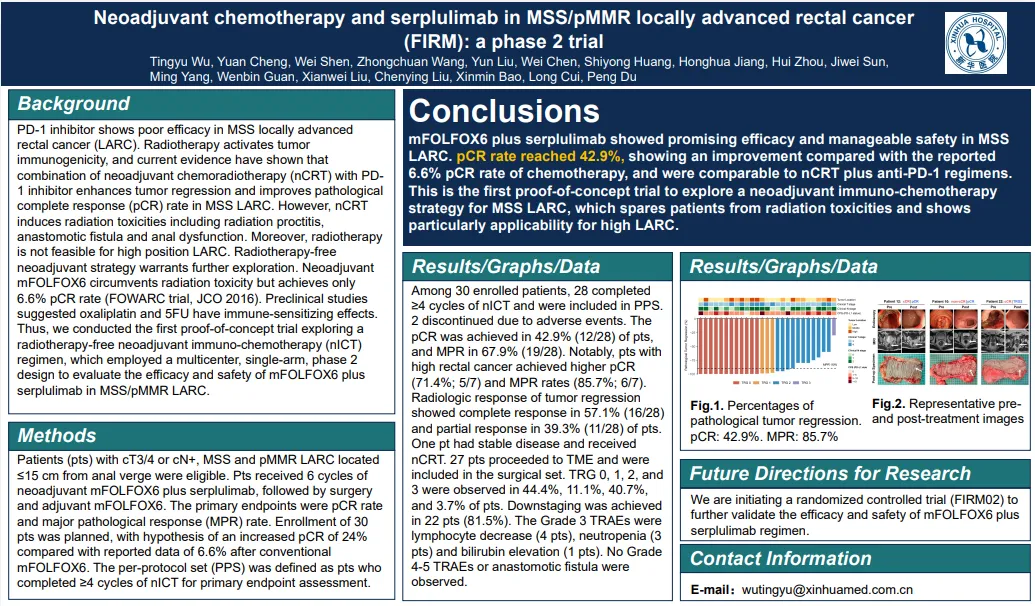
新辅助化疗联合斯鲁利单抗用于 MSS/pMMR 局部晚期直肠癌(FIRM 研究):一项 Ⅱ 期临床试验
试验设计:
入组cT3/4或cN+,距肛缘≤15 cm,MSS/pMMR的 LARC患者(pts),接受6个周期的新辅助mFOLFOX6和斯鲁利单抗,随后进行手术和辅助mFOLFOX6。主要终点为pCR率和主要病理反应(MPR)率
结果:
在30例入组患者中,28例完成了≥4个nICT周期并纳入PPS。2例因不良事件停用。pCR是在42.9% (12/28), MPR 67.9%(19/28)。高位LARC(距肛缘10 cm,不适合nCRT)患者的pCR和MPR率分别为71.4%和85.7%
结论:
mFOLFOX6联合斯鲁利单抗在MSS LARC中显示出良好的疗效和可管理的安全性。pCR率出乎意料地达到42.9%,与化疗报道的6.6% pCR率相比有所改善,与nCRT +抗pd -1方案相当。这是探索MSS LARC的nICT策略的第一个概念验证试验,该策略使患者免于放疗毒性,并特别适用于高位LARC。
关于复宏汉霖
复宏汉霖(2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已在全球获批上市10款产品,5个上市申请分别获中国药监局和欧盟EMA受理。自2010年成立以来,复宏汉霖已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,公司商业化生产基地已相继获得中国、欧盟和美国GMP认证。
复宏汉霖前瞻性布局了一个多元化、高质量的产品管线,涵盖约50个分子,并全面推进基于自有抗PD-1单抗H药 汉斯状®的肿瘤免疫联合疗法。截至目前,公司已获批上市产品包括全球首个获批一线治疗小细胞肺癌的抗PD-1单抗汉斯状®(斯鲁利单抗,欧洲商品名:Hetronifly®)、自主研发的中美欧三地获批单抗生物类似药汉曲优®(曲妥珠单抗,美国商品名:HERCESSI™,欧洲商品名:Zercepac®)、国内首个生物类似药汉利康®(利妥昔单抗)、地舒单抗生物类似药Bildyos®和Bilprevda®,以及帕妥珠单抗POHERDY®。公司亦同步就19个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
Results from Neoadjuvant studies of Henlius' serplulimab in colon/rectal cancer released at ASCO GI 2026
The 2026 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI) was held from January 8 to 10 in San Francisco, United States. At this conference, the results of two neoadjuvant studies of Henlius' innovative anti-PD-1 monoclonal antibody(mAb) HANSIZHUANG (serplulimab, trade name in Europe: Hetronifly®), in the field of MSS/pMMR locally advanced colon/rectal cancer were officially released in the form of posters, further highlighting the therapeutic potential of serplulimab in the field of gastrointestinal cancers. Serplulimab is the world's first and only anti-PD-1 mAb to have succeeded in a phase 3 perioperative gastric cancer registration study and is also the world's first anti-PD-1 mAb for first-line treatment of small cell lung cancer, achieving groundbreaking progress in both lung and gastric cancers.
In the field of gastrointestinal cancers, in addition to the approved esophageal squamous cell carcinoma (ESCC) indication, serplulimab is the first drug to receive Breakthrough Therapy Designation from the National Medical Products Administration (NMPA) Center for Drug Evaluation (CDE). As the world’s first perioperative regimen for gastric cancer to replace adjuvant chemotherapy with immunotherapy monotherapy, its New Drug Application (NDA) has been accepted by the CDE and granted Priority Review. Besides, an international, multicenter Phase 3 trial (ASTRUM-015) of serplulimab in combination with bevacizumab and chemotherapy as first-line therapy for metastatic colorectal cancer (mCRC) has completed patient enrollment, potentially addressing the clinical gap in first-line immunotherapy for non-MSI-H mCRC. At this conference, positive results of serplulimab from two investigator-initiated trials (IITs) for neoadjuvant therapy in locally advanced colon/rectal cancer were released. The data indicate that serplulimab in neoadjuvant therapy is expected to improve the chances of radical surgery and long-term survival rates for patients with locally advanced high-risk colon cancer, while also showing potential to provide a radiation-free treatment regimen for patients with locally advanced rectal cancer.
Serplulimab demonstrates unique advantages in treating various solid tumors via its differentiated mechanism. The drug not only induces stronger PD-1 internalization—reducing PD-1 receptor presence on T cells for rapid and potent immune activation [1]—but also minimizes PD-1-mediated recruitment of the co-stimulatory molecule CD28, thereby preserving CD28 signaling [2-4], enhancing downstream AKT activity [5], and promoting sustained T-cell activation. Focused on gastrointestinal cancers and lung cancer, serplulimab has been approved for the treatment of squamous non-small cell lung cancer (sqNSCLC), extensive-stage small cell lung cancer (ES-SCLC), ESCC, and non-squamous non-small cell lung cancer (nsqNSCLC). Up to date, it has been approved in over 40 countries and regions including China, the U.K., Germany, Singapore, and India, covering nearly half of the global population and accelerating global accessibility. The results of 4 pivotal trials of serplulimab were published in the Journal of the American Medical Association (JAMA), Nature Medicine, Cancer Cell and British Journal of Cancer, respectively. It has also received orphan drug designations granted by the US FDA, the European Commission, Swissmedic, Korea MFDS and Mexico COFEPRIS.
The results of the two studies released at this ASCO GI conference are as follows:
Title: Neoadjuvant short-course radiotherapy combined with CAPOX and PD-1 inhibitor for MSS/pMMR high-risk locally advanced colon cancer: A randomized, prospective, multicentre, phase II trial (TORCH-C).
Study design: This is a prospective, multicenter, randomized phase II trial (NCT05732493). A total of 120 LACC (T4/bulky N+M0,pMMR/MSS) patients will be randomized to either a chemotherapy group or a radiotherapy group. The chemotherapy group receives 4 cycles of CAPOX. The radiotherapy group receives SCRT(25Gy in 5 fraction)and 4 cycles of the PD-1 inhibitor (serplulimab) combined with CAPOX. The radiotherapy target volume includes only the primary colon tumor and enlarged lymph nodes, without elective nodal irradiation. After neoadjuvant therapy, patients will be evaluated for radical colon resection. The primary endpoint is pathological complete response (pCR) rate. The secondary endpoints include tumor downstaging, R0 resection, 3-year disease free survial (DFS), 3-year overall survival(OS), 3-year local recurrence-free survival and treatment-related toxicity
Results: As of August 31, 2025, 120 patients have been enrolled and randomized. Of thses, 79 patients have completed neoadjuvant treatment and surgery (43 in the chemotherapy group, 36 in the radiotherapy group). All 79 patients achieved radical resection, with R0 resection rates of 95.3% (41/43) in the chemotherapy group (95.3%, 41/43), and 97.2% (35/36) in the radiotherapy group. The pCR rate (TRG 0) was 11.6% (5/43) in chemotherapy group and 47.2% (17/36) in the radiotherapy group. The most common grade 3-4 adverse event (AE) among patients completing neoadjuvant treatment was thrombocytopenia (15.3%, 15/98), including 5 cases of grade 4 thrombocytopenia.
Conclusion: The combination of PD-1 inhibitor, SCRT, and chemotherapy shows promising efficacy and may significantly improve pCR rates in patients with MSS/pMMR highrisk LACC. This regimen may provide a new therapeutic option to achieve R0 resection and improve long-term survival.
Title: Neoadjuvant chemotherapy and serplulimab in MSS/pMMR locally advanced rectal cancer (FIRM): a phase II trial
Study design: Patients (pts) with cT3/4 or cN+, MSS and pMMR LARC located ≤15 cm from anal verge were eligible. Pts received 6 cycles of neoadjuvant mFOLFOX6 and serplulimab, followed by surgery and adjuvant mFOLFOX6. The primary endpoints were pCR rate and major pathological response (MPR) rate.
Results: Among 30 enrolled patients, 28 completed ≥4 cycles of nICT and were included in PPS. 2 discontinued due to adverse events. The pCR was achieved in 42.9% (12/28) of pts, and MPR in 67.9% (19/28). For high LARC (>10 cm from anal verge, ineligible for nCRT), the pCR and MPR rates were 71.4% and 85.7%.
Conclusion: mFOLFOX6 plus serplulimab showed promising efficacy and manageable safety in MSS LARC. pCR rate unexpectedly reached 42.9%, showing an improvement compared with the reported 6.6% pCR rate of chemotherapy, and were comparable to nCRT plus anti-PD-1 regimens. This is the first proof of concept trial to explore a nICT strategy for MSS LARC, which spares pts from radiation toxicities and shows particularly applicability for high LARC.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases and ophthalmic diseases. To date, 10 products have been approved for marketing across multiple countries and regions, and 5 marketing applications have been accepted for review in China and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centre and Shanghai-based commercial manufacturing facilities certificated by China, the EU and U.S. GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering about 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as the backbone. To date, the company's launched products include HANSIZHUANG (serplulimab, trade name: Hetronifly® in Europe), the world’s first anti-PD-1 mAb for the first-line treatment of SCLC, HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANLIKANG (rituximab), the first China-developed biosimilar, denosumab Bildyos® and Bilprevda®, and pertuzumab Poherdy®. What’s more, Henlius has conducted over 30 clinical studies for 19 products, expanding its presence in major markets as well as emerging markets.
To learn more about Henlius, visit https://www.henlius.com/en/index.html and connect with us on LinkedIn at https://www.linkedin.com/company/henlius/.

 研发追踪
研发追踪
 复宏汉霖
复宏汉霖  2026-01-13
2026-01-13
 65
65

 研发追踪
研发追踪
 细胞与基因治疗领域
细胞与基因治疗领域  2026-01-13
2026-01-13
 62
62

 研发追踪
研发追踪
 药明康德
药明康德  2026-01-12
2026-01-12
 147
147